Figure 1 (left) and Figure 2
Peter Ruddock, Contributor
The games of the 29th Olympiad have just concluded, and what a magnificent spectacle they were! An abundance of world records, a super medal haul and the incredible performance of our athletes, especially Usain Bolt, made these Olympics extra special for Jamaica.
Results like these are probably long overdue and many fans feel that athletes were cheated in the past by competitors using banned drugs. Technology is levelling the playing field, however, and the detection of illegal substances is getting easier. From the list of banned substances, steroids are oftentimes the drugs of choice.
As part of my chemistry PhD studies at the University of the West Indies, Mona, I made and used steroids. Not for biological use, however, as they were never taken into the body (verified from my 'not very muscular' 5' 11', 160-lb frame). Rather, my research focused on using steroids to study new reactions. The results of such studies can lead to new medicines and materials.
ATOMIC STRUCTURE
While they have received a lot of negative press over the years, steroids are actually essential for life. Tens of steroids are made naturally by the human body. Too low or too high levels can lead to illness and death. Steroids play an important function, such as aiding sperm production, muscular growth, pregnancy, the repair of inflammation in the joints and the flow of salts in and out of cells. Some of the more common steroids include testosterone (male sex hormone), oestrogen (female sex hormone), vitamin D and cholesterol.
Almost all steroids in the body are made from cholesterol, which is made in the body from fats. Thus, although in many minds cholesterol, a greasy yellow substance, is linked with clogged arteries, heart disease and death, without it, there would be no human life.
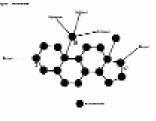
A single steroid molecule is exceedingly small and cannot be seen with the naked eye. An ounce (28 grams) of steroid would fit in a teaspoon but contains about six sextillion (6 followed by 21 zeros) individual molecules. A microscope powerful enough to examine an individual steroid would show a structure made up largely of carbon, hydrogen and oxygen atoms. Seventeen or more carbons join together to make up the 'skeleton' of the steroid while hydrogen and oxygen 'flesh' out the frame. The carbons link together to form four fused rings. (See Figure 1).
Three of the rings have six sides while the fourth has five sides. The 19-carbon steroid in Figure 1 is that of the male sex hormone, testosterone. Oxygens are attached to carbon 3 and carbon 17. Twenty-eight hydrogen atoms also form part of the structure, but for clarity of illustration, only three are shown in the diagram on carbon 19.
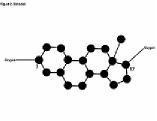
Tiny changes in its 'carbon skeleton' cause monumental changes in the steroid's effect on the body. For example, while the structure of the male hormone testosterone has 19 carbons and 28 hydrogens, the female hormone estradiol (Figure 2) has 18 carbons and 24 hydrogens. The change includes the loss of carbon 19 and its hydrogens. On that tiny difference, one carbon and four hydrogens, hinges the distinction between the male and female of the species.
STEROIDS IN MEDICINE
Chemists, especially those in medicinal/pharmaceutical research, take advantage of the powerful effect that these small changes produce. Researchers modify the carbon skeleton by adding or removing carbons, hydrogens, oxygens and other atoms. This produces many different steroids, which are then tested. They find application in many areas of life, from fertility treatments to contraceptives, from anabolic steroids to arthritis, from asthma to eczema. Cholesterol even finds use, as in the field of liquid-crystal displays.
Steroids are an immensely important group of compounds, necessary for the existence of life itself. They are oftentimes abused, but I hope you can agree, deserving of a lot more respect than they have been given.
As an aside, if one superimposes the steroid rings on to the Olympic rings, a rather snug fit becomes apparent. A Freudian slip? By design? Your guess is as good as mine.
Feedback may be sent to peter.ruddock@yahoo.com or columns@gleanerjm.com.

