Vallana Hill, Contributor
Ethanol is a clean-burning, high-octane fuel that is produced from renewable sources. At its most basic, ethanol is grain alcohol, produced from crops such as straw, corn and sugar cane. Pure 100 per cent ethanol is not generally used as a motor fuel; instead, a percentage of ethanol is combined with unleaded gasoline. This is beneficial because the ethanol: decreases the fuel's cost , increases the fuel's octane rating and decreases gasoline's harmful emissions. Any amount of ethanol can be combined with gasoline, but the most common blends are: E10 (10 per cent ethanol and 90 per cent unleaded gasoline) and E85 (85 per cent ethanol and 15 per cent unleaded gasoline). E10 is approved for use in any make or model of vehicle sold in the US while E85 is an alternative fuel for use in flexible fuel vehicles (FFVs).
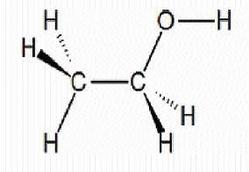
It is widely used in cars in many countries because it is easy to manufacture and process and is an increasingly common alternative to gasoline in many parts of the world, more recently Jamaica. Ethanol is a volatile, flammable, colourless liquid that has a strong characteristic odour. It burns with a smokeless blue flame that is not always visible in normal light. The physical properties of ethanol stem primarily from the presence of its hydroxyl group and the shortness of its carbon chain. Ethanol's hydroxyl group is able to participate in hydrogen bonding, rendering it more viscous and less volatile than less polar organic compounds of similar molecular weight. Ethanol is classified as a primary alcohol, meaning that the carbon to which its hydroxyl group is attached has at least two hydrogen atoms attached to it as well.
The basic steps for large scale production of ethanol are: (1) fermentation of sugars, (2) distillation, (3) dehydration and (4) denaturing which is optional. Prior to fermentation, some crops require the conversion of carbohydrates such as cellulose and starch into sugars through a process of hydrolysis or saccharification in which enzymes are used to convert starch into sugar.
Fermentation
Ethanol fermentation is the biological process by which sugars such as glucose, fructose, and sucrose are converted into cellular energy, thereby producing ethanol and carbon dioxide as metabolic waste products. Yeasts carry out ethanol fermentation on sugars in the absence of oxygen. Ethanol fermentation is responsible for the rising of bread dough, the production of ethanol in alcoholic beverages, and for much of the production of ethanol for use as fuel.
Distillation
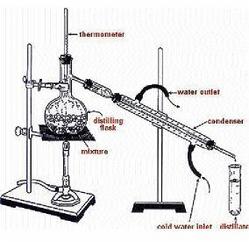
For the ethanol to be usable as a fuel, water must be removed. Most of the water is removed by distillation, a process by which mixtures are separated based on their level of volatility.
www.chemheritage.org/EducationalServices/pharm/antibiot/activity/distil/distil07.gif
The ethanol-water mixture is boiled and condensed, but since ethanol has a lower volatility, it would evaporate first, thereby effectively separating the two. The purity is limited to 95 or 96 per cent due to the formation of a low-boiling water-ethanol. The 95.6 per cent m/m (96.5 per cent v/v) ethanol, 4.4 per cent m/m (3.5 per cent v/v) water mixture may be used as a fuel alone, but this, is immiscible in gasoline, so the water fraction is typically removed in further treatment in order to burn in combination with gasoline in gasoline engines.
Dehydration
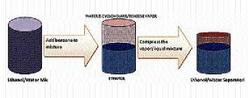
There are basically five dehydration processes to remove the water from an ethanol/water mixture. The first process, used in many early fuel ethanol plants, is called azeotropic distillation and consists of adding benzene or cyclohexane to the mixture. When these components are added to the mixture, it forms a heterogeneous mixture in the form of a vapour-liquid-liquid equilibrium, which when distillated produces anhydrous ethanol on the bottom, and a vapour mixture of water and cyclohexane/benzene on top. When condensed, this becomes a two-phase liquid mixture.
After this point the ethanol can be consumed as E100 - 100 per cent ethanol or mixed with gasoline to form any other graded fuel mixture.
Ethanol is known to burn more cleanly and have less CO2 emissions than regular gasoline. Most modern cars are suited for the E10 octane rating, however for higher grades, special engines are required. The higher compression ratios in an ethanol-only engine allow for increased power output and better fuel economy than could be obtained with lower compression ratios. In general, ethanol-only engines are tuned to give slightly better power and torque output than gasoline-powered engines. However, for cars that traditionally ran on gasoline, it can be noted that the increased volatility of the E10 mixture will cause the depletion of the fuel at an increased rate, while some cars are known to be incompatible with the ethanol mixture. The fact that is it renewable energy makes it better in curbing the dependency on oil, and it contributes less pollution and greenhouse gas emissions.
See if your car is compatible you can check the website: www.onlinemechanical.net/ethanol-E10.html
The MIAS is a non-profit organisation of the University of the West Indies, Pure and Applied Sciences Department, offering analytical, technical and, web services and specialised science projects. If you have any question or comments about these articles please Email: mias@uwimona.edu.jm or contact the MIAS Analytical Services Division at 970-2042 or 512-3067 for enquires on services offered.